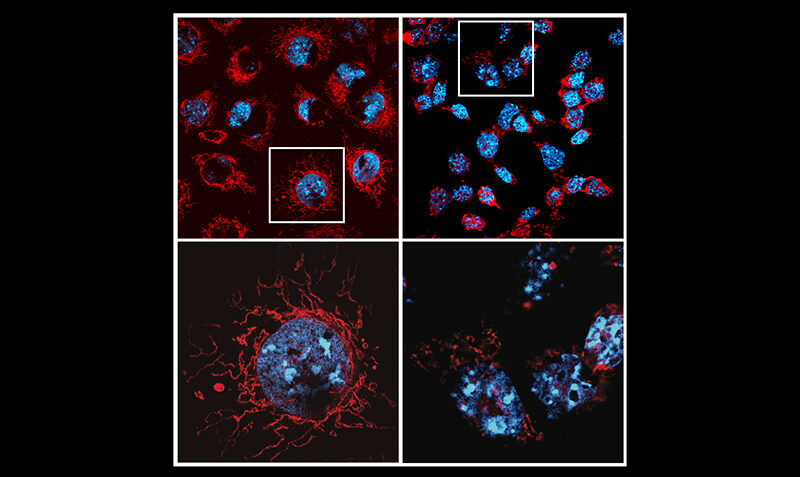
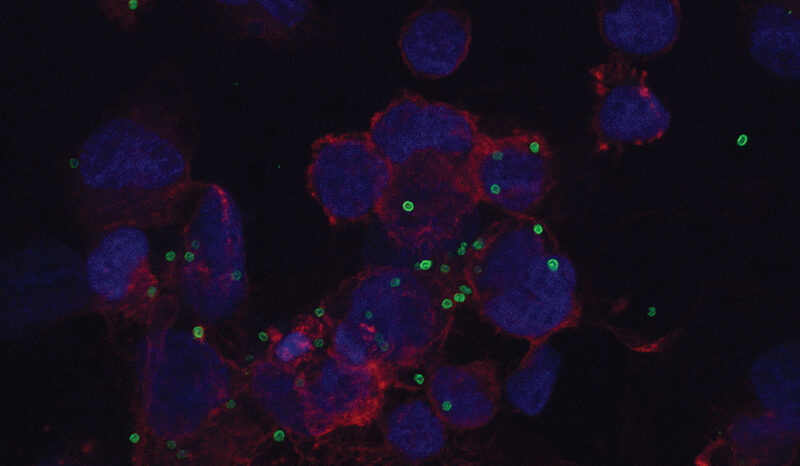
The research strengthens the potential of MCL-1 as a cancer drug target, which is currently the subject of clinical trials all over the world.
While drug compounds targeting MCL-1 that have been developed to date are considered extremely effective at combating cancer, they have unfortunately also caused significant side effects in early clinical trials, particularly in the heart.
Co-senior researcher Professor Andreas Strasser said the findings could help resolve the safety issues of drugs targeting MCL-1 that have hindered these promising treatments.
“If we can direct MCL-1 inhibitors preferentially to tumour cells and away from the cells of the heart and other healthy tissues, we may be able to selectively kill cancer cells while sparing healthy tissues,” Prof Strasser, a WEHI laboratory head, said.
The study also lays the groundwork for better combination therapies. By understanding the distinct pathways the protein influences, researchers can design smarter dosing strategies and pair MCL-1 inhibitors with other treatments to reduce toxicity.
“This work exemplifies the power of discovery science,” said co-senior researcher Professor Marco Herold, CEO of the Olivia Newton-John Cancer Research Institute (ONJCRI).
“The sophisticated preclinical models we developed allow us to interrogate the precise function of MCL-1, and to address fundamental biological questions that have direct relevance to human disease.”