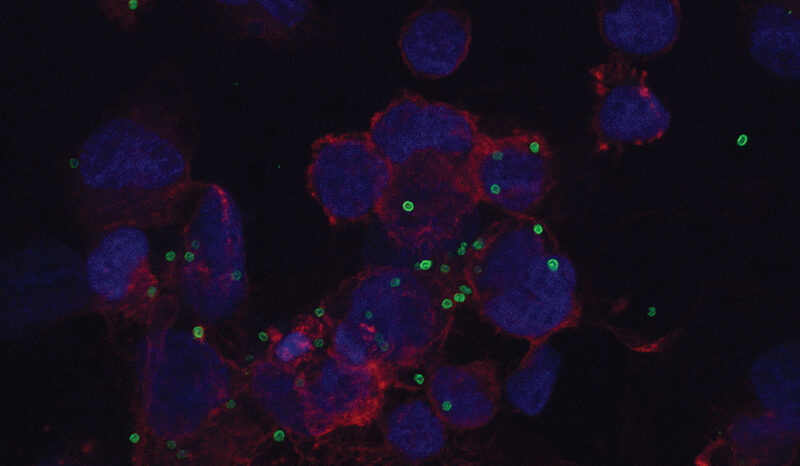
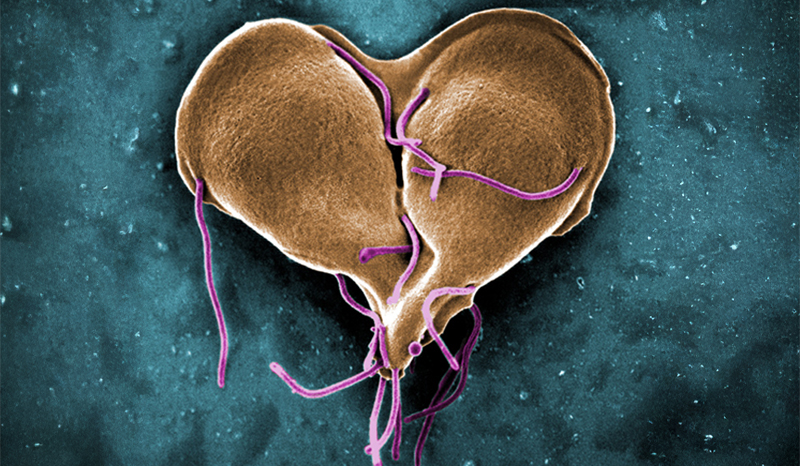
Research by the University of Melbourne’s Professor Damian Purcell, Head of Molecular Virology at the Doherty Institute and co-lead author of the study, isolated the virus from First Nations donors and identified significant genetic differences between the HTLV-1c strains from Central Australia compared to the HTLV-1a strains found internationally.
The new findings show that both HTLV-1 strains cause disease in mice, with HTLV-1c showing more aggressive features. The identified drug therapies were found to be equally effective against both strains.
Prof Purcell and Associate Prof Lloyd Einsiedel worked with the National Aboriginal Community Controlled Health Organisation (NACCHO) HTLV-1 committee and the Australian Department of Health over many years to advocate for guidance on HTLV-1 from the World Health Organization (WHO) that led to them formally classify the virus as a Threatening Pathogen to Humans in 2021.
This resulted in the development of formal WHO policies to reduce transmission internationally and the development of clinical management guidelines for HTLV-1c in Central Australia under NACCHO leadership.
“Despite Australia’s high burden of HTLV-1, the virus and its associated diseases are still not notifiable in most states and true infection rates in the nation remain unknown,” Prof Purcell said.
“People at risk from HTLV-1 deserve biomedical tools like those that provide game-changing therapeutic and prevention options for other blood-borne persistent viral infections, such as HIV.
“There is a real opportunity to prevent the transmission of HTLV-1 and end the diseases caused by these infections. Our research findings are a major leap forward in this.”
The research team is currently in talks with the companies behind the HIV antivirals used in this study, to see if HTLV-1 patients can be included in their ongoing clinical trials. If successful, this would pave the way for these drugs to become the first approved pre-exposure prophylaxis against HTLV-1 acquisition.
These findings are supported by the Australian Centre for HIV and Hepatitis Virology Research, The Phyllis Connor Memorial Trust, Drakensberg Trust and the National Health and Medical Research Council (NHMRC).
–
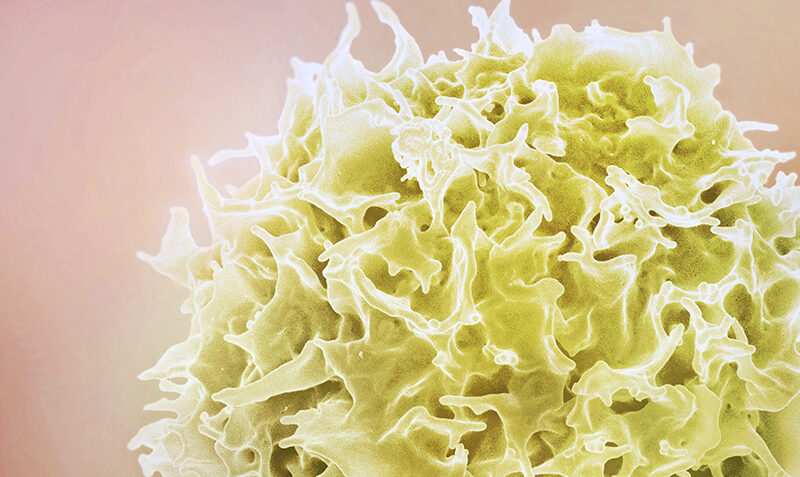
Header image: HTLV-1 is a virus that infects a type of white blood cell called a T‐lymphocyte, or T-cell (pictured). The new study could lead to the first treatments and potential cure for the virus that impacts around 10 million people each year. Credit: National Institute of Allergy and Infectious Diseases.