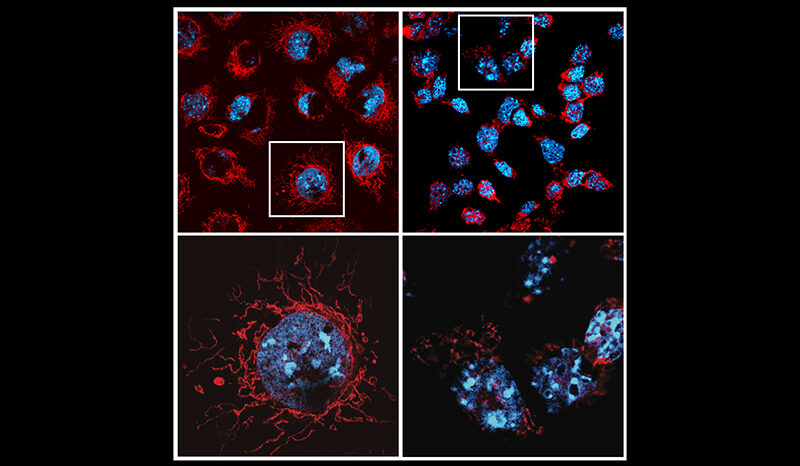
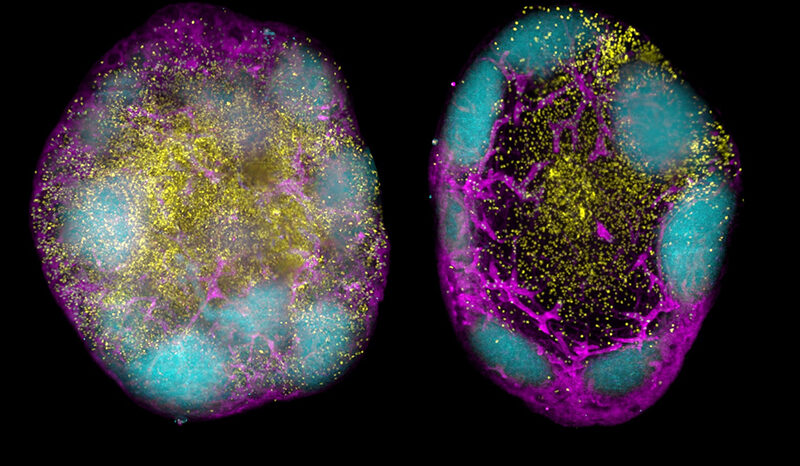
When it works properly, the p53 protein serves as a powerful defence mechanism against cancer development by ensuring that cells with compromised DNA either get repaired or removed from our body.
Mutations to this protein can be triggered by a variety of environmental factors – such as exposure to UV radiation – if the body’s cell division process goes awry, or if they are genetically inherited.
These abnormalities can lead to a dysfunctional protein that has lost the crucial ability to regulate cellular responses that prevent tumour development and growth. This is known as loss-of-function.
Additionally, some researchers believe these mutations can also lead to a supercharged protein that helps cancerous cells survive and proliferate, known as gain-of-function.
In a new study, published in Cancer Discovery, researchers from WEHI and Trento University (Italy) have answered an age-old question by proving which function helps mutant p53 fuel tumour growth.
Associate Professor Gemma Kelly, a co-corresponding author of the paper, said understanding how exactly these mutations contribute to cancer is critical to understanding how to treat patients bearing cancers with the dysfunctional proteins.
“Our study has provided the first evidence to show that it is actually the loss-of-function that impacts cancer growth. We found no evidence of gain-of-function contributing to cancer growth,” Assoc Prof Kelly, a Laboratory Head in WEHI’s Blood Cells and Blood Cancer Division, said.
“This is significant because until now, there was not much evidence available to properly show whether it is the protein’s loss or gain-of-function that spurs cancer growth.
“If you look at all cancers of humanity, about 50% of them have a mutation in p53. Specific cancers like those of the pancreas, lung and breast, commonly have defects in these proteins.”