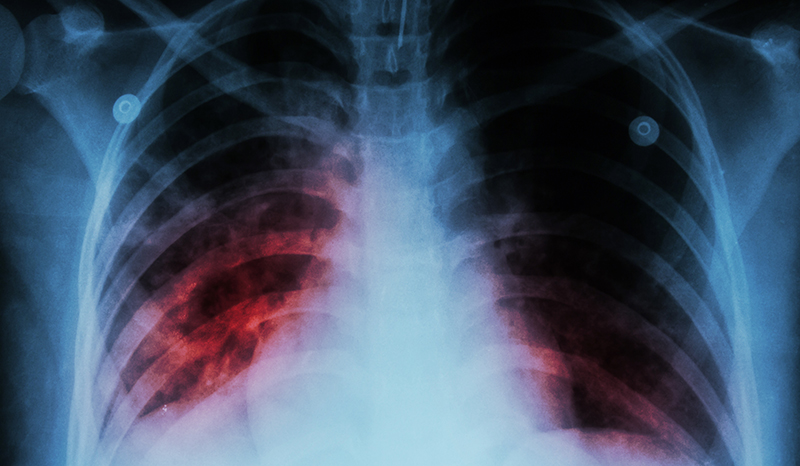
Tuberculosis (TB) remains humanity’s longest pandemic infection, and the leading single cause of global infectious mortality. As humans have co-evolved with Mycobacterium tuberculosis (Mtb), most adults and children develop natural protection – only 5-15% of those infected ever develop disease, generally within 5 years, although it can take decades. With an estimated 2 billion people having been infected globally this results in 10 million people diagnosed with TB and over 1 million deaths, annually. Our inability to eliminate TB is due to its immunological manifestations profoundly challenging our fundamental understanding of what constitutes and mediates protective immunity and disease risk. There is also currently have no diagnostic test that can identify individuals infected with Mtb, and critically, who of those is most at risk of developing TB and thus should be prioritised to receive treatment.
The Coussens Lab studies how the response of innate immune cells, namely macrophages and neutrophils, that engulf the TB causing bacteria is dysregulated by biosocial and co-morbid factors that increase risk of TB development. We combine analysis of clinical cohort samples to identify novel pathways of pathogenesis in humans, with in vitro infection models to determine the molecular mechanism underlying disease risk. Together this enables us to identify novel therapeutic targets and develop diagnostic biomarkers to improve earlier TB diagnosis and inform who will most benefit from treatment.
We achieve this using a range of cutting-edge techniques to interrogate genetic, epigenetic, transcriptomic and proteomic changes in both the host and bacteria to identify how these impact the inflammatory response during infection. To study the regulation of cell death pathways and the heterogeneity of cellular responses due to tissue micro-environmental changes we probe these using single cell omics, spatial omics and advanced live cell imaging techniques.