Immune checkpoints are the brakes of the immune system, allowing immune responses to be switched off after a threat – such as a virus infection – is over. Without these brakes, uncontrolled immune responses can cause inflammatory tissue damage and autoimmune disease.
However, some cancer cells take advantage of these brakes, using them to switch off immune cells that would otherwise destroy the tumours.
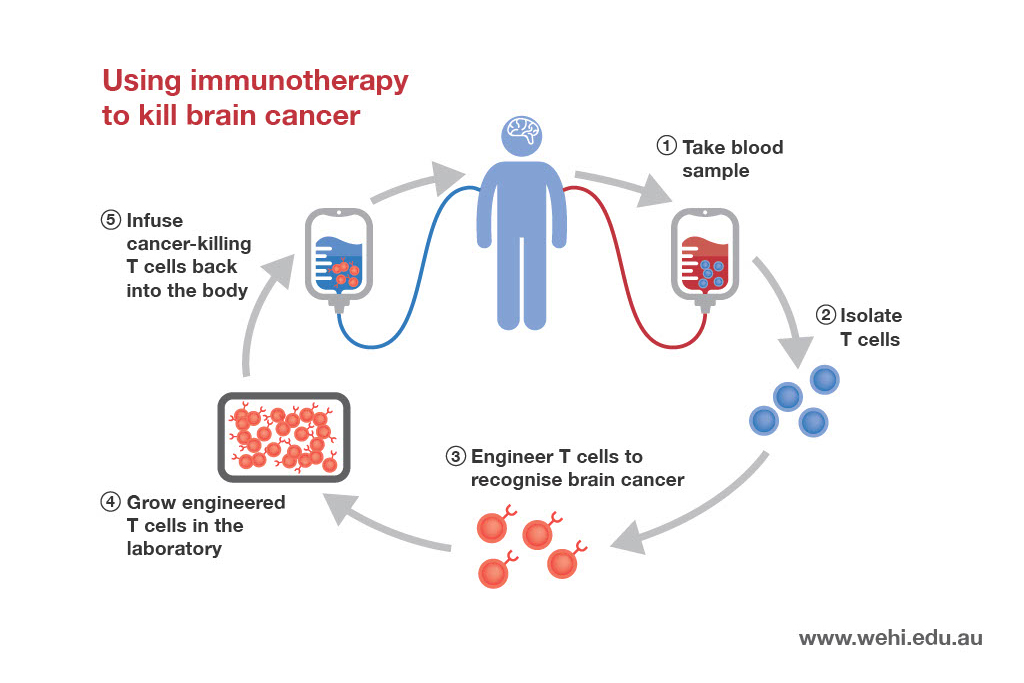
Immune checkpoint inhibitors release the brakes, enabling immune cells to attack tumours.
Some of the most exciting new cancer therapies are known as anti-PD1 and anti-CTLA4 immunotherapies. Our researchers are studying these immunotherapies in several preclinical models of cancer, including lung, stomach and breast cancer.
This research could help to identify patients who may benefit from these immunotherapies and lead to future clinical trials aimed at improving patient outcomes.