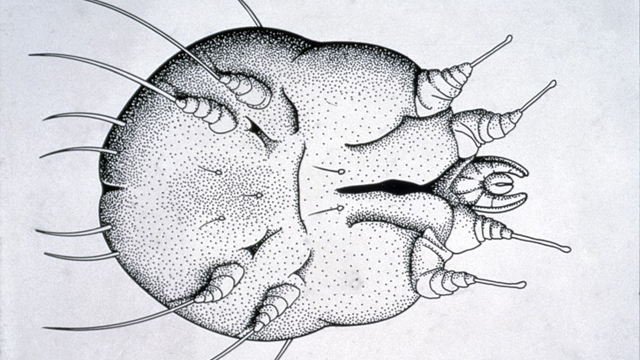
Scabies is a skin infection caused by the microscopic scabies mite, Sarcoptes scabiei. Female mites burrow into the top layers of human skin to lay eggs. This causes irritation, a rash and intense itching, which is particularly bad at night.
Scabies mites release proteins that suppress immune reactions in the skin. These can lead to bacterial skin infections and sores. Bacteria carried by scabies mites and excessive scratching in people with scabies may also contribute to skin infections. Often people with scabies also have trouble sleeping. This can make it hard for them to work, or to attend school.
Scabies mites are spread between people by:
- Prolonged skin-to-skin contact
- Sharing of clothes and bedding
- Living in overcrowded conditions
It is estimated that around 300 million people worldwide are infected with scabies. It occurs in all communities. Scabies, and the complications of scabies infection, are especially prevalent in overcrowded and resource-poor communities in tropical areas.
In Australia, some remote Aboriginal communities have very high rates of scabies. Up to half of the children in some communities are infected. These scabies infections begin at a young age, on average, at two months. They may have lifelong recurrences.
People with scabies in remote Aboriginal communities frequently have skin sores infected with group A streptococcus (‘group A strep’) and Staphylococcus aureus bacteria. These bacterial infections can lead to acute rheumatic heart disease and chronic kidney disease. These serious health burdens can potentially be reduced by preventing scabies infections in a community.
Most people with scabies are infected with only five to 10 mites. People with a weakened immune system, including the elderly and those with conditions such as AIDS, can develop a disease called crusted scabies. In this severe form of infection, a person can carry millions of mites. People with crusted scabies can be debilitated and disfigured. They may be socially isolated and avoid medical attention. People with crusted scabies often act as a ‘reservoir’ for scabies infection in their community. They can re-infect those around them who may have previously been treated and cured of their infection.