Victorian brain cancer researchers have achieved a global-first, using an innovative process to learn how a new drug suppresses tumour activity and provides hope to patients with low-grade gliomas (LGG).
It’s the first clinical trial to be conducted through the pioneering Brain Perioperative platform, or “BrainPOP”, which is led by The Brain Cancer Centre and funded by the Victorian Government.
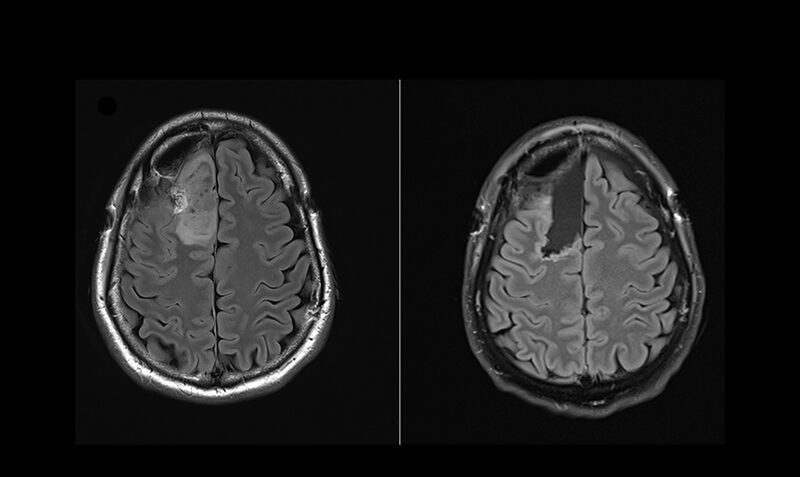
LGGs are a slow-growing type of brain cancer that significantly impact the lives of patients—many of whom are young adults in their prime. Characterised by a specific mutation in a gene called IDH, current treatments are limited and LGG have long been considered incurable.
But a new treatment is on the horizon, thanks to the discovery of mutations in LGG and a new and innovative process for treating them.
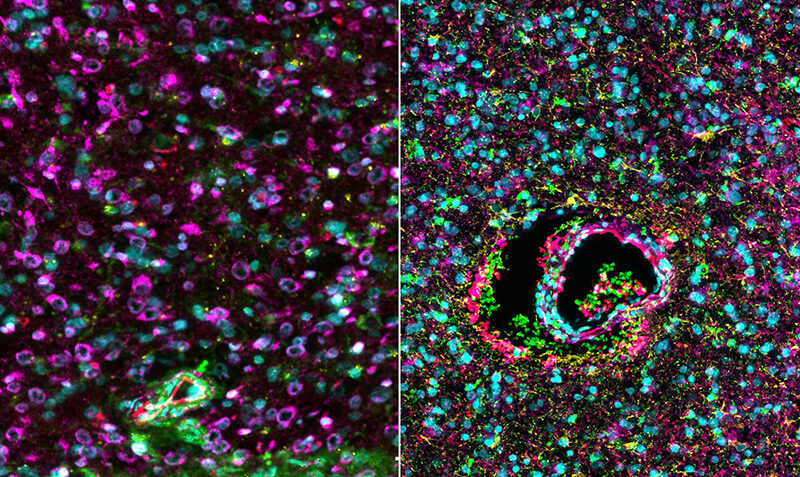
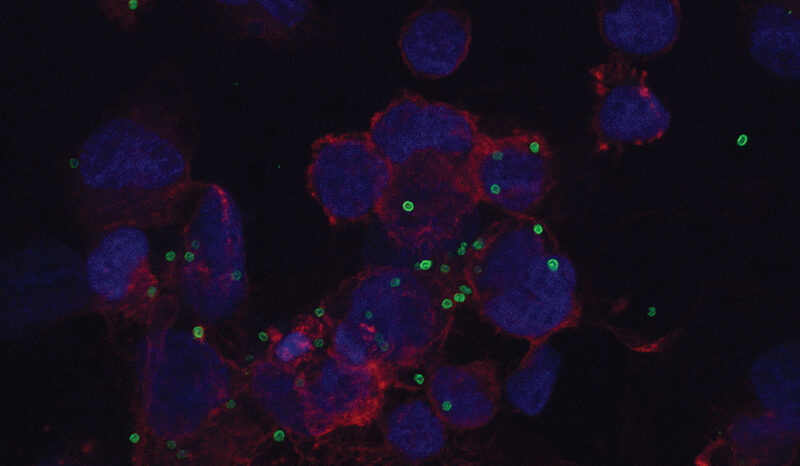
Using a drug called Safusidenib – an oral inhibitor targeting the mutated IDH1 gene – researchers from the Royal Melbourne Hospital (RMH), WEHI and the Peter MacCallum Cancer Centre (Peter Mac) observed the drug’s effect on LGGs tumour samples both before and after treatment.
And the results have been promising, with the proof of principle study published in the prestigious journal Nature Medicine.
“We want Victorian brain tumour patients to have care that is equal to anywhere in the world,” Professor Kate Drummond, Director of Neurosurgery at the RMH and the trial’s lead investigator, said.
“This trial is not only a revolution in the way we test new treatments but brings new opportunities for this most deserving group of patients with a devastating disease.”
Prof Drummond said the response from patients had been overwhelmingly positive, with many excited to be part of the innovative trial, “even though the trial required two operations and intensive treatment”.
“Brain cancer patients are desperate for new treatments, and clinical trials like this are exactly what is needed,” Prof Drummond said.