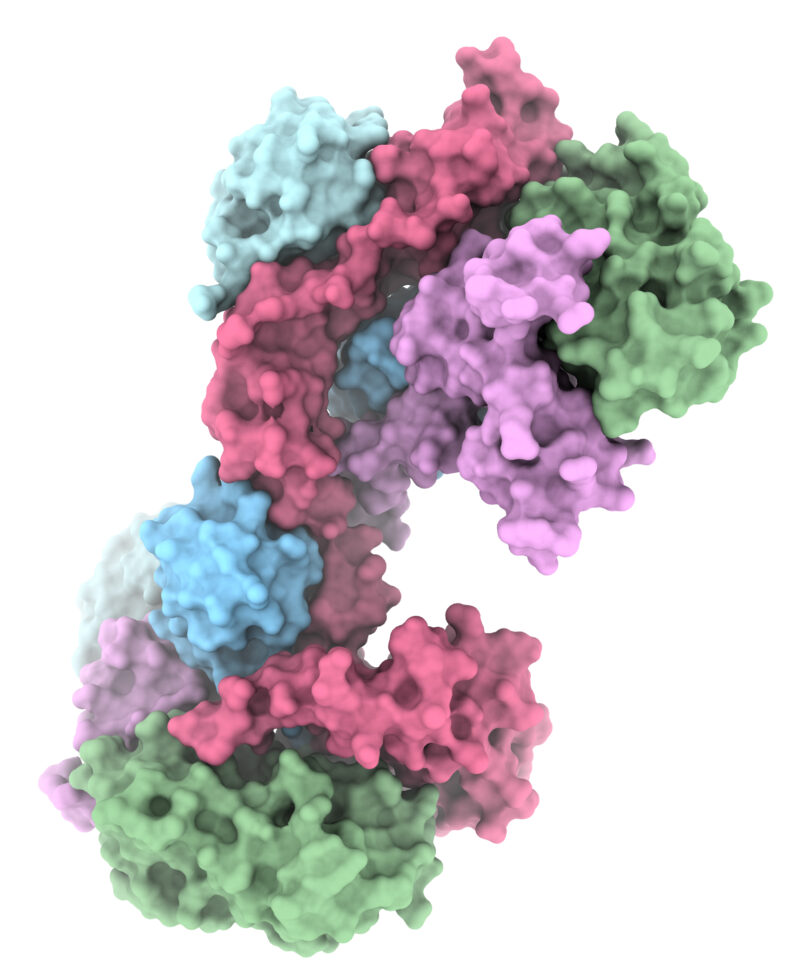
The Lechtenberg lab investigates E3 ubiquitin ligases, enzymes that catalyse a cellular process termed ubiquitination. E3 ubiquitin ligases are molecular label makers that attach the small protein ubiquitin to other proteins to induce protein degradation or regulate protein activity, subcellular localisation or interactions with other proteins. Ubiquitination provides a diverse signalling code and regulates almost all processes inside the human cell and is a central player in human physiology and disease.
Our research provides the molecular basis for E3 ubiquitin ligase activity and regulation and aims to transfer these insights to the cellular function of the E3s ligases, for example in ribosomal quality control, innate immune signalling or cancer. Our lab utilises a combination of structural (crystallography, cryo-EM), biophysical, biochemical and cell biological methods.