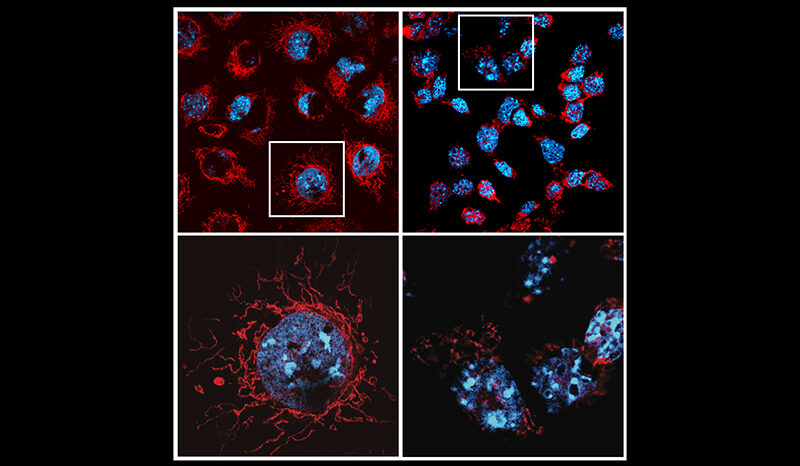
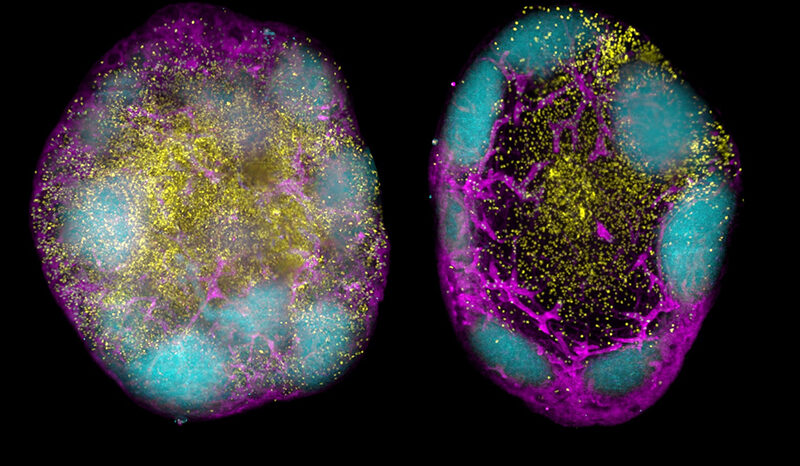
Mitochondria produce energy at a cellular level in all living things, and cells that require a lot of energy can contain hundreds or thousands of mitochondria. The PARK6 gene encodes the PINK1 protein, which supports cell survival by detecting damaged mitochondria and tagging them for removal.
In a healthy person, when mitochondria are damaged, PINK1 gathers on mitochondrial membranes and signals through a small protein called ubiquitin, that the broken mitochondria need to be removed. The PINK1 ubiquitin signal is unique to damaged mitochondria, and when PINK1 is mutated in patients, broken mitochondria accumulate in cells.
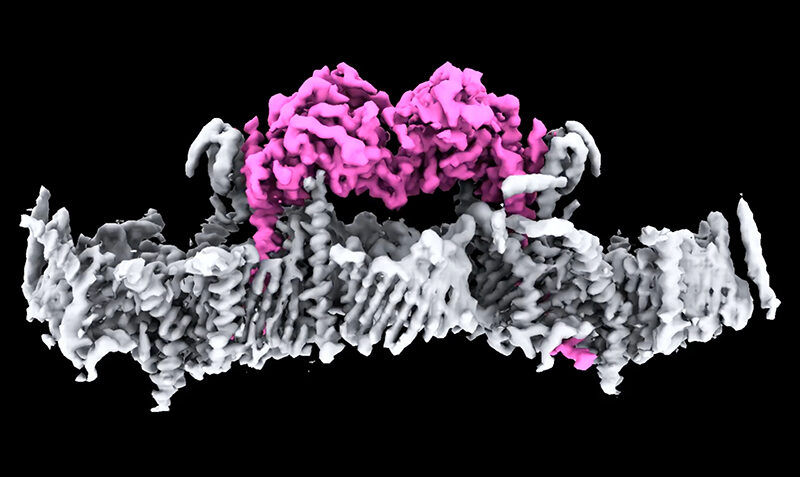
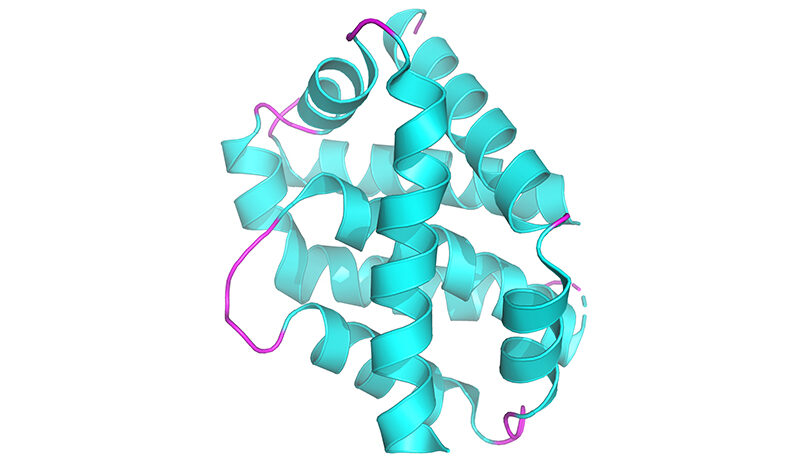
Although PINK1 has been linked to Parkinson’s, and in particular Young Onset Parkinson’s Disease, researchers had been unable to visualise it and did not understand how it attaches to mitochondria and is switched on.
Corresponding author on the study and head of WEHI’s Ubiquitin Signalling Division, Professor David Komander, said years of work by his team have unlocked the mystery of what human PINK1 looks like, and how it assembles on mitochondria to be switched on.
“This is a significant milestone for research into Parkinson’s. It is incredible to finally see PINK1 and understand how it binds to mitochondria,” said Prof Komander, who is a laboratory head in the WEHI Parkinson’s Disease Research Centre.
“Our structure reveals many new ways to change PINK1, essentially switching it on, which will be life-changing for people with Parkinson’s.”