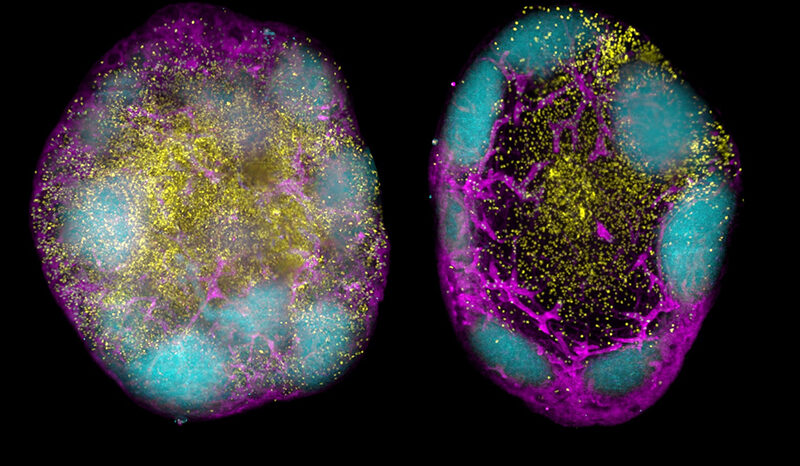
PINK1 is a mitochondrial protein that supports cell survival. When it is mutated, it can no longer function properly.
Mitochondria are often described as the powerhouses of cells because they produce adenosine triphosphate (ATP), the source of energy at a cellular level.
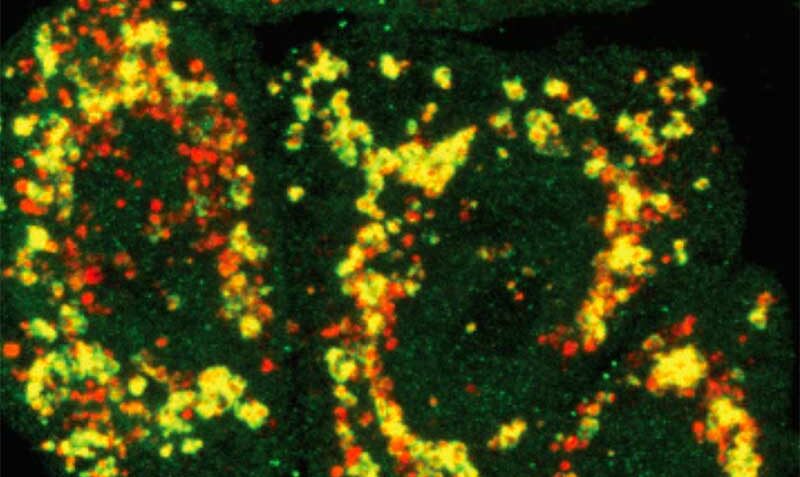
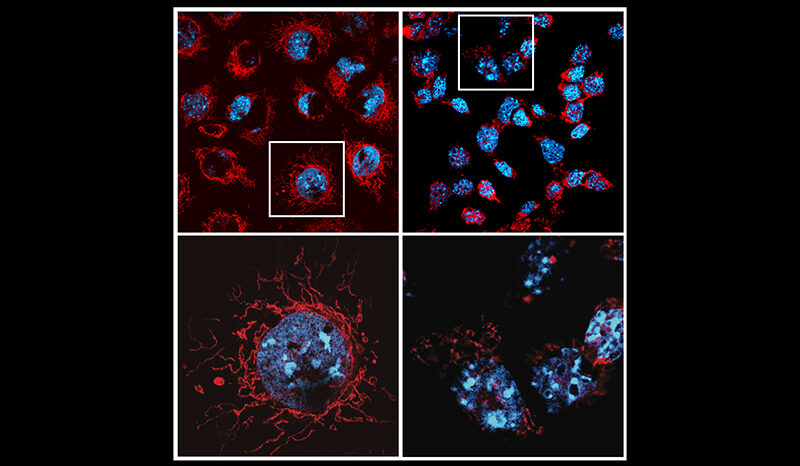
When mitochondria are damaged and need disposing to keep cells healthy, they are first tagged by PINK1.
Building a better understanding into the pathways of how damaged mitochondria are processed is crucial to developing a drug therapy for Parkinson’s disease. Research into PINK1 is one such pathway.
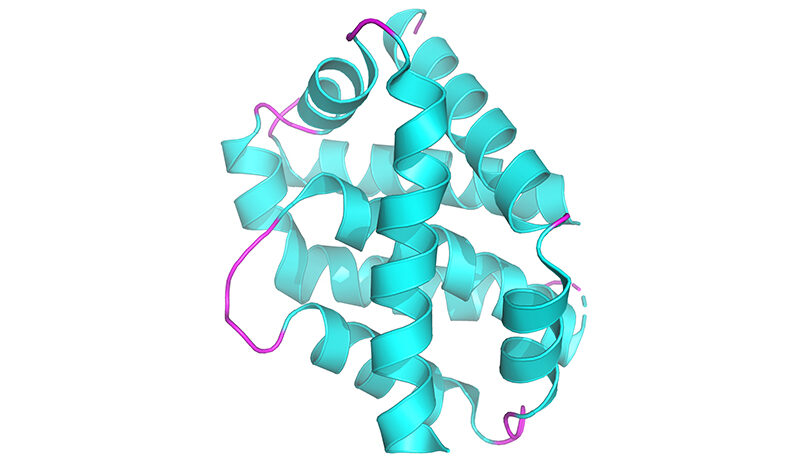
In the new study, researchers developed a method of imaging PINK1 at an atomic level using high powered microscopy. The team then tracked how the protein switches into its active form when ATP, or its “copycat” molecule KTP, are added.
KTP was reported over a decade ago to enhance PINK1 activity in cells and had represented a promising strategy for early onset Parkinson’s disease treatment.
But the research team, led by Dr Zhong Yan Gan (now at the Laboratory of Molecular Biology, Cambridge), Dr Sylvie Callegari and Professor David Komander discovered that KTP could not bind to the protein as previously thought.
Dr Callegari is a Senior Researcher in the Komander Lab whose work contributes to the progress being made by WEHI’s Parkinson’s Disease Research Centre.
“While we don’t fully understand how PINK1 is stabilised on mitochondria yet, we know that PINK1 tagging is the first step in identifying mitochondrial damage,” she said.
A critical step in this process is the activation of the signal-generating ability of PINK1, as soon as it has accumulated on mitochondria.
This requires an additional ingredient, the molecule ATP, which binds to and is used by PINK1 to generate the damage signal.