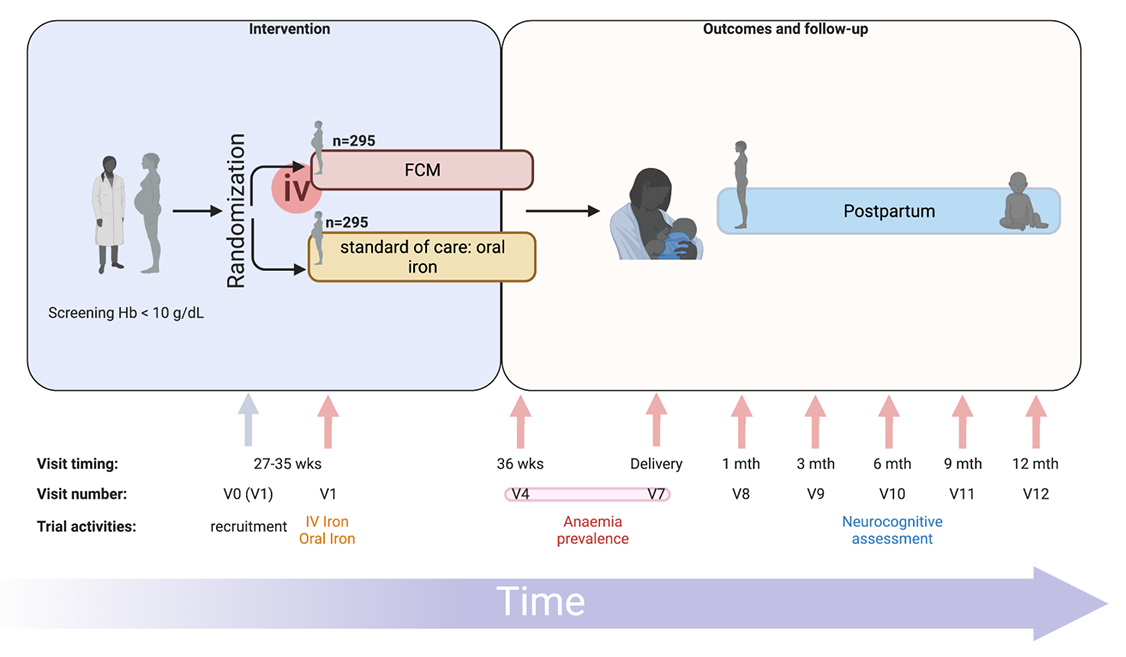
Professor Kamija Phiri, Principal Investigator, Training and Research Unit of Excellence, College of Medicine
Professor Sant-Rayn Pasricha, Principal Investigator, Population Health and Immunity Division, WEHI
Dr Martin Mwangi, Trial Manager, Training and Research Unit of Excellence, College of Medicine
Dr Ricardo Ataide, Trial Manager, Population Health and Immunity Division, WEHI
Ernest Moya, Team Coordinator, Training and Research Unit of Excellence, College of Medicine
Dr Zinani Truwah, Team Coordinator, Training and Research Unit of Excellence, College of Medicine
Dr Glory Mzembe, Team Coordinator, Training and Research Unit of Excellence, College of Medicine
Maclean Vokhiwa, Neuro Assessment Coordinator, Training and Research Unit of Excellence, College of Medicine
Dr Louise Randall, Neuro Study Coordinator, Population Health and Immunity Division, WEHI
Sabine Braat, Senior Biostatistician, Population Health and Immunity Division, WEHI
Dr Rebecca Harding, Trial Statistician, Population Health and Immunity Division, WEHI
Phoebe Fitzpatrick, Data Manager, Population Health and Immunity Division, WEHI
Mphatso Mwambinga, Data Officer, Training and Research Unit of Excellence, College of Medicine
William Nkhono, Data Officer, Training and Research Unit of Excellence, College of Medicine
Naomi Von Dinklage, Program Coordinator, Population Health and Immunity Division, WEHI
Dr Khic-Houy Prang, Implementation Science Researcher, The University of Melbourne
Ebony Verbunt, Implementation Science Researcher, The University of Melbourne
Dr Lucinda Manda-Taylor, Implementation Science Researcher, Kamuzu University of Health Sciences
Dr Effie Chipeta, Implementation Science Researcher, Kamuzu University of Health Sciences
Professor Gary Rance, Audiologist, the University of Melbourne
Dr Peter Carew, Audiologist, The University of Melbourne
Dr Sumie Leung, Cognitive Neuroscientist, The University of Melbourne
Associate Professor Stefan Bode, Cognitive Neuroscientist, The University of Melbourne
Associate Professor Katherine Johnson, Developmental Cognitive Neuroscientist, The University of Melbourne
Dr Peter Simm, Paediatric Endocrinologist and Honorary Fellow, Hormone Research, Murdoch Children’s Research Insititute
Associate Professor Marc Seal, Principal Research Fellow & Group Leader, Developmental Imaging, Murdoch Children’s Research Institute