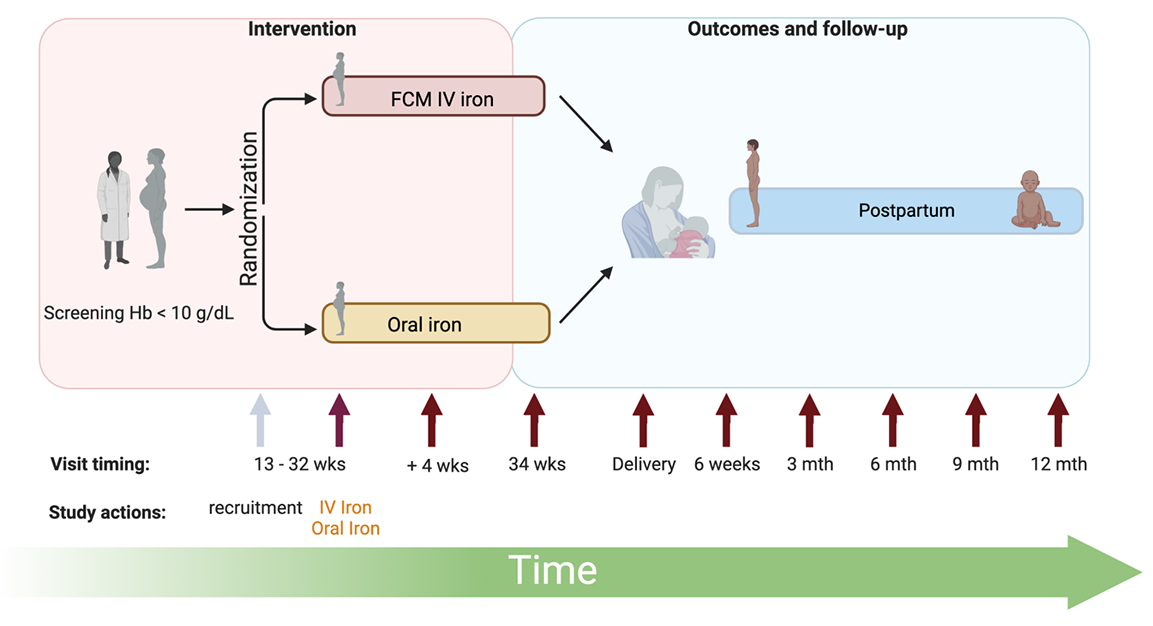
Professor Sant-Rayn Pasricha, Principal Investigator, Population Health and Immunity Division, WEHI
Dr Jena Hamadani, Principal Investigator, Maternal and Child Health Division, International Centre for Diarrhoeal Disease Research, Bangladesh
Dr Imrul Mohammed Hasan, Trial Manager, Maternal and Child Health Division, International Centre for Diarrhoeal Disease Research, Bangladesh
Dr Eliza Davidson, Trial Manager, Population Health and Immunity Division, WEHI
Dr Alistair McLean, Trial Statistician, Population Health and Immunity Division, WEHI
Dr Rebecca Harding, Trial Statistician, Population Health and Immunity Division, WEHI
Sabine Braat, Senior Biostatistician, Population Health and Immunity Division, WEHI
Phoebe Fitzpatrick, Data Manager, Population Health and Immunity Division, WEHI
Naomi Von Dinklage, Program Coordinator, Population Health and Immunity Division, WEHI
Dr Louise Randall, Neuro Study Coordinator, Population Health and Immunity Division, WEHI
Dr Khic-Houy Prang, Implementation Science Researcher, The University of Melbourne
Ebony Verbunt, Implementation Science Researcher, The University of Melbourne
Dr Bidhan Krishna Sarker, Implementation Science Researcher, International Centre for Diarrhoeal Disease Research, Bangladesh
Dr Shamim Ahmed, Trial Manager, International Centre for Diarrhoeal Disease Research, Bangladesh
Dr Quaiyum Rahman, Team Coordinator, International Centre for Diarrhoeal Disease Research, Bangladesh
Dr Saiful Alam Bhuiyan, Team Coordinator, International Centre for Diarrhoeal Disease Research, Bangladesh
Jahiduj Jaman, Data Team Coordinator, International Centre for Diarrhoeal Disease Research, Bangladesh
Shamima Shiraji, Developmental Assessment Team Coordinator, International Centre for Diarrhoeal Disease Research, Bangladesh