Our research has provided new insight into the fundamental cell processes of apoptosis and mitophagy and their role in disease.
Key discoveries include:
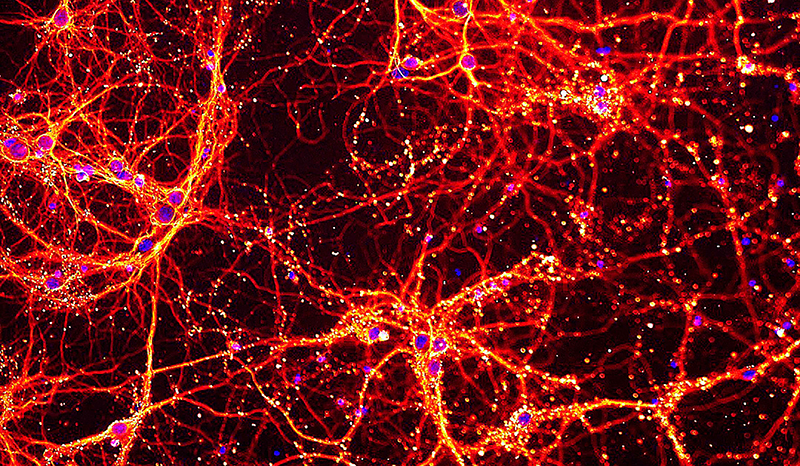
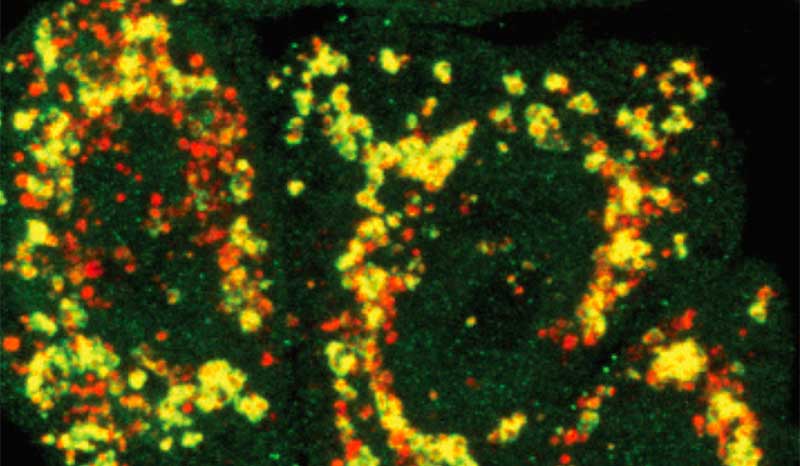
We have made key discoveries in the control of PINK1/Parkin mediated mitophagy (Bernardini et al 2018 EMBO J, Gan et al Nature 2022).
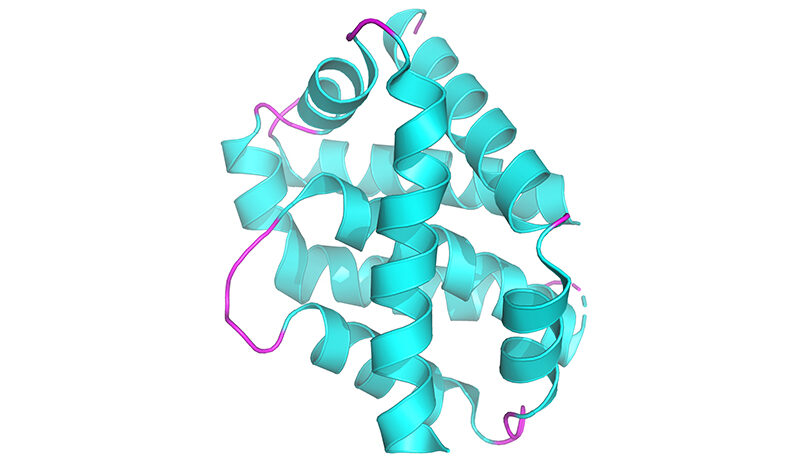
Characterised how Bak and Bax function during apoptosis that is now the accepted model of apoptotic pore formation (Dewson et al Molecular Cell 2008, Dewson et al CD&D 2012, Dewson et al Molecular Cell 2009, Ma..Dewson JBC 2013).
In collaboration with the Czabotar lab at WEHI provided the first crystal structures of Bax:BH3-only protein and Bax and Bak homodimers (Czabotar et al Cell 2013, Brouwer et al Molecular Cell, 2014 and 2017).
Identified novel aspects of apoptosis regulation and avenues for therapeutic intervention (Chin et al Nature Comms 2018; Ma et al CD&D 2014; Van Delft et al Nature Chem Biol 2019).